
Follow this link to find news coverage, public statements from The Institute for Human Gene Therapy, FDA warning letters, Office for Human Research Protections determination letters, and an update on Paul.
This page has civil settlement agreements, information on NIH grants awarded to Drs. Wilson, Raper, and Batshaw, links to transcripts of meetings of the National Institutes of Health Recombinant DNA Advisory Committee, and related documents.
Phase I Pilot Study of Liver-Directed Gene Therapy for Partial Ornithine Transcarbamylase Deficiency. Study chairs or principal investigators: Mark Batshaw M.D. Sponsors: National Institute of Child Health and Human Development (NICHD) and Children's National Medical Center. Record first received on 1999-10-18: http://www.clinicaltrials.gov/ct/show/NCT00004386?order=2
Phase I Study of Adenoviral Vector Mediated Gene Transfer for Ornithine Transcarbamylase in Adults With Partial Ornithine Transcarbamylase Deficiency. Sponsors: FDA Office of Orphan Products Development and the University of Pennsylvania. Study chairs or principal investigators: Steven Raper M.D. Record first received on 1999-10-18: http://www.clinicaltrials.gov/ct/show/NCT00004498?order=1
One consequence of Jesse's death – and his father's persistent dedication to research protection advocacy – was the creation of a public adverse event database for gene transfer studies. This isn't enough.
NIH Recombinant DNA Advisory (RAC) Committee. Meeting Minutes. 1995-06-08 and 09: http://oba.od.nih.gov/oba/rac/minutes/6-8-9-95.pdf
NIH Recombinant DNA Advisory (RAC) Committee. Meetings and Conferences: http://oba.od.nih.gov/rdna_rac/rac_meetings.html
A Phase I Study of Adenoviral Vector Mediated Gene Transfer to Liver in Adults with Partial Ornithine Transcarbamylase Deficiency. Protocol 9512-139. Post-mortem. Minutes of the NIH Recombinant DNA Advisory (RAC) Committee. 1999-12-08 through 10: http://oba.od.nih.gov/oba/rac/minutes/1299rac.pdf
Data Management Report: http://oba.od.nih.gov/oba/rac/SAE_rpts/mod1299s.pdf
Protocol Amendments, Updates: http://oba.od.nih.gov/oba/rac/amendments/Mod399a.pdf
NIH Recombinant DNA Advisory (RAC) Committee Working Group on Current Issues in Adverse Event Reporting. Post-mortem/Fallout, Minutes. 2000-03-08 through 10: http://oba.od.nih.gov/oba/rac/minutes/RACmin3-00.pdf
Data Management Report: http://oba.od.nih.gov/oba/rac/SAE_rpts/mgtrpt3-00.pdf
Initial Review of A Phase I Study of Adenoviral Vector Mediated Gene Transfer to Liver in Adults with Partial Ornithine Transcarbamylase Deficiency. Protocol 9512-139. Minutes of the NIH Recombinant DNA Advisory (RAC) Committee. 1995-12-04 and 05: http://oba.od.nih.gov/oba/rac/minutes/124-5-95.pdf
Mark Batshaw M.D. Report of subject death in Protocol 9512-139. 1999-09-20. Safety Reports and Adverse Events for Human Gene Transfer Protocols. NIH Recombinant DNA Advisory Committee Meeting Minutes. 1999-12-08 through 10: http://oba.od.nih.gov/oba/rac/minutes/1299rac.pdf
Data Management Report: http://oba.od.nih.gov/oba/rac/SAE_rpts/mod1299s.pdf
Protocol Amendments, Updates: http://oba.od.nih.gov/oba/rac/amendments/Mod1299a.pdf
NIH Office of Biotechnology Activities
Frequently Asked Questions (FAQs) about the NIH Review Process for Human Gene Transfer Trials. NIH Recombinant DNA Advisory (RAC) Committee.
NIH Guidance on Informed Consent For Gene Transfer Research. NIH Recombinant DNA Advisory (RAC) Committee.
Educational Materials. NIH Recombinant DNA Advisory (RAC) Committee. (Selection of articles and other resources)
FDA Cellular and Gene Therapy Guidances
Disclosure of Information OTC Gene Therapy 1999-12-01
NIH/FDA Genetic Modification Clinical Research Information System (GeMCRIS), Index of Information
GeMCRIS Public Information Site Search
Scientific Abstract, A Phase I Study of Adenoviral Vector Mediated Gene Transfer to Liver in Adults with Partial Ornithine Transcarbamylase Deficiency. Protocol 9512-139. Available from http://www.gemcris.od.nih.gov/Abstracts/139_s.pdf
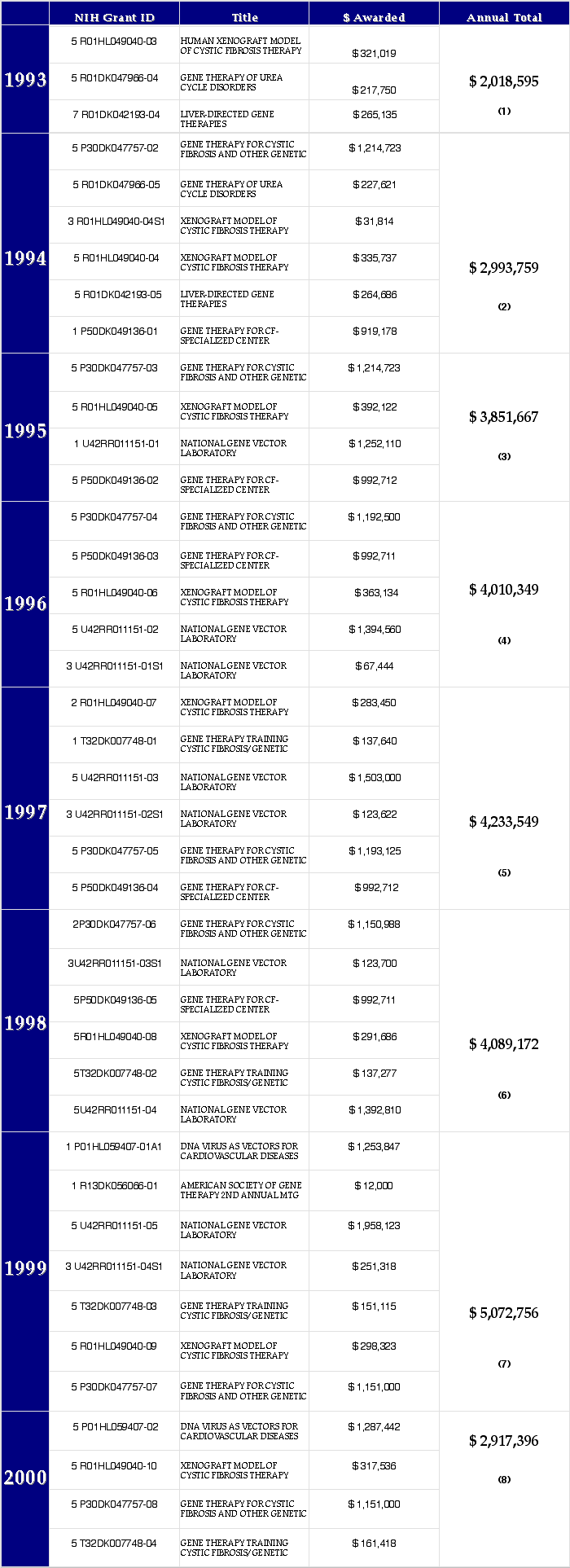
NIH Funds Awarded 1993-2000: $29,187,243 (not a complete list)
Notes
1. 1993 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/FY1993.pennsylv.txt
2. 1994 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/FY1994.pennsylv.txt
3. 1995 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/FY1995.pennsylv.txt
4. 1996 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/FY1996.pennsylv.txt
5. 1997 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/FY1997.pennsylv.txt
6. 1998 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/fy1998.pennsylv.txt
7. 1999 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/fy1999.pennsylv.txt
8. 2000 NIH awards accessed 2005-02-27 at http://grants1.nih.gov/grants/award/state/fy2000.pennsylv.txt
2010-04-09: these links no longer work; we appreciate your patience while we update them.
2010-04-09: these links no longer work; we appreciate your patience while we update them.
2010-04-09: these links no longer work; we appreciate your patience while we update them.
Accessed 2005-02-27 from Computer Retrieval of Information on Scientific Projects (CRISP)
U.S. Settles Case of Gene Therapy Study That Ended With Teen's Death. United States Attorney's Office Press Release. 2005-02-09: http://web.archive.org/web/20050214120445/http://www.justice.gov/usao/pae/News/Pr/2005/feb/UofPSettlement+release.html
Mark Batshaw M.D., and Children's National Medical Center (CNMC). U.S. Department of Justice Settlement Agreement. 2005-02-09: http://www.circare.org/foia3/batshawsettlement.pdf
Institute for Human Gene Therapy, University of Pennsylvania. U.S. Department of Justice Settlement Exhibit. Promoting Safety in Clinical Research at the University of Pennsylvania.
2005-02-09: http://www.circare.org/foia3/ihgt_exhibittosettlement.pdf
Institute for Human Gene Therapy, University of Pennsylvania. U.S. Department of Justice Settlement Agreement. 2005-02-09: http://www.circare.org/foia3/IHGT_settlement_5.pdf
Steven Raper M.D. U.S. Department of Justice Settlement Agreement. 2005-02-09: http://www.circare.org/foia3/raper_settlement.pdf
Steven Raper M.D. U.S. Department of Justice Settlement Exhibit 1: Medical Monitor. 2005-02-09: http://www.circare.org/foia3/RAPER_exhibit_2.pdf
James Wilson M.D, Ph.D. U.S. Department of Justice Settlement Agreement. 2005-02-09: http://www.circare.org/foia3/wilson5_settlementagreement.pdf
James Wilson, M.D., Ph.D. U.S. Department of Justice Settlement Exhibit 1: Medical Monitor. 2005-02-09: http://www.circare.org/foia3/wilson_exhibit2.pdf
Last Updated: 2010-04-09
If you find the information on this page helpful please support CIRCARE with a tax-deductible contribution today. Because CIRCARE doesn't accept funds from pharmaceutical or medical device manufacturers, we depend on contributions from individuals like you to help us advocate for meaningful protection of human subjects in research. Find out more on our Support page
All material on this site © CIRCARE Incorporated (2002- ) or as indicated. Single copies can be downloaded for personal education. Adobe Reader™ :: ::