
 What's Wrong With This Research
What's Wrong With This Research
 Study Documents
Study Documents  Regulatory Correspondence
Regulatory Correspondence
 Funding and Sponsorship
Funding and Sponsorship  Media Articles
Media Articles
 Malariotherapy Research Publications
Malariotherapy Research Publications
 Research on Malaria and HIV
Research on Malaria and HIV
 Credibility
Credibility  FOIA: UCLA, NIH and John Fahey, M.D.
FOIA: UCLA, NIH and John Fahey, M.D.  Varia
Varia
NB: editor's comments = Arial font gray text.
Currently (2005-01-17) this notice appears in a solicitation for charitable donations on the web site of the Deaconess Foundation Inc., ostensibly an entity related to Deaconess Associations Inc., parent of the Heimlich Institute Foundation:
The Heimlich Institute
With your support, the Heimlich Institute can continue its important research into treatment for HIV/AIDS, cancer, cystic fibrosis, asthma and many other health conditions. We already have evidence that these methods work, but further studies are needed to substantiate these procedures and treatments. The Institute, which is a non-profit organization, depends on funds from free-thinking individuals and organizations to continue its commitment to saving lives. (1)
Some people would agree that free-thinking
is an inapposite description for healthcare corporations that disdain the opinion of the regulator, or are otherwise oblivious to widely accepted ethical guidelines for the conduct of research with fellow human beings.
As outlined in the table below, it seems as if Dr. Heimlich, the Heimlich Institute Foundation Inc., and its parent corporation, Deaconess Associations Inc., have not been involved with the malariotherapy research on subjects with HIV/AIDS formerly co-conducted with Dr. Xiao Ping Chen in Guangzhou China for many years. In fact, the only malariotherapy study acknowledging funding from the Heimlich Institute was published in 1997.
During the period between 1997 and 2003, however:
AIDS research and educationin excess of $220,000.
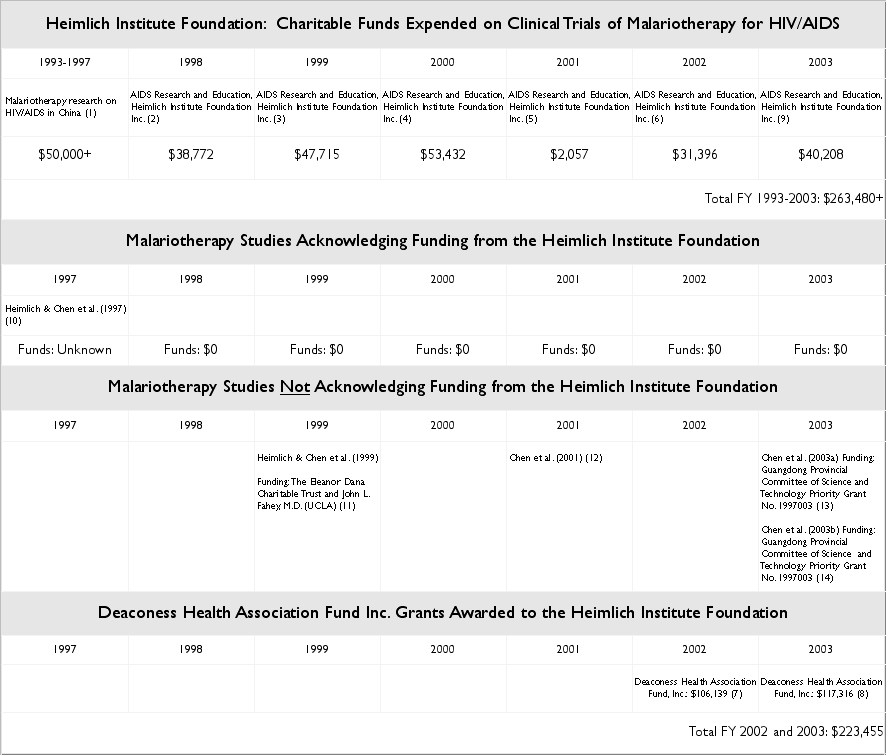
Notes, Funding & Sponsorship Chart
1. Heimlich's Audacious Maneuver. Pamela Warrick. The Los Angeles Times. 1994-10-30:
Actress Amy Irving gave $50,000. Other major supporters included actress Estelle Getty, high-powered agent Sandy Bresler and ventriloquist Paul Winchell, according to Heimlich's foundation and other sources. Fueled by hundreds of thousands of dollars from such celebrity donations, Heimlich researchers have begun injecting a small group of HIV-positive men in China with malaria-infected blood.
2. Heimlich Institute Foundation Inc. IRS Form 990 FY 1998, p. 2. Accessed from Guidestar.org on 2004-12-05 at: http://www.guidestar.org/
3. Heimlich Institute Foundation Inc. IRS Form 990 FY 1999, p. 2. Accessed from Guidestar.org on 2004-12-05 at: http://www.guidestar.org/
4. Heimlich Institute Foundation Inc. IRS Form 990 FY 2000, p. 2. Accessed from Guidestar.org on 2004-12-05 at: http://www.guidestar.org/
5. Heimlich Institute Foundation Inc. IRS Form 990 FY 2001, p. 2. Accessed from Guidestar.org on 2004-12-05 at: http://www.guidestar.org/
6. Heimlich Institute Foundation Inc. IRS Form 990 FY 2002, p. 2. Accessed from Guidestar.org on 2004-12-05 at: http://www.guidestar.org/
7. Deaconess Health Association Fund Inc. IRS Form 990 FY 2002. Part II, Schedule of Grants and Allocations, p. 15. There is nothing to indicate these funds were or were not spent on malariotherapy for HIV/AIDS research. Accessed from Guidestar.org on 2004-12-05 at: http://www.guidestar.org/
8. Deaconess Health Association Fund Inc. IRS Form 990 FY 2003. Part II, Schedule of Grants and Allocations, p. 15. There is nothing to indicate these funds were or were not spent on malariotherapy for HIV/AIDS research. Accessed from Guidestar.org on 2005-06-22 at: http://www.guidestar.org/
9. Heimlich Institute Foundation Inc. IRS Form 990 FY 2003, p. 2. Accessed from Guidestar.org on 2005-03-04 at: http://www.guidestar.org/
10. Heimlich HJ, Chen XP, Xiao BQ, Liu SG, Lu YH, Spletzer EG, Yao JL. Malariotherapy for HIV patients. Mechanisms of Ageing and Development 1997;93(1-3):84. Accessed on 2007-04-13 at: http://web.archive.org/web/20040209070512/www.heimlichinstitute.org/malariohiv.html
Acknowledgements: Protocol, data management and information retrieval were supplied by the Heimlich Institute. Patient selection and care, and laboratory measurements were provided by the Municipal Health and Anti-Epidemic Station and the Yishou Hospital, both of Guangzhou. We thank the staffs of these institutions for their assistance.
11. Chen XP, Heimlich, HJ., Xiao, BQ., Liu, SG., Lu, YH., Yao, J., Spletzer, EG. Phase-1 Studies of Malariotherapy for HIV Infection. Chin Med Sci J. 1999;14(4):228. Accessed on 2005-09-10 at: http://www.bioethicswatch.org/foia/imt_cjms1999.pdf
12. Chen XP. et al. Impact of Acute Vivax Malaria on the Immune System of HIV-Positive Subjects. Poster No. 149. AIDS Vaccine. 2001. Accessed on 2005-09-10 at: http://web.archive.org/web/20030721190418/http://63.84.172.40/Posters/149.1.a.pdf
13. Chen XP, Xiao BQ, Xu H, Shi W, Gao K, Rao J. Procedure and clinical assessments of malariotherapy: recent experience in 20 HIV patients. Chin Med J. 2003;116(7):1016-21. Accessed on 2007-04-13 at: http://www.cmj.org/Periodical/PDF/2003/200371016.pdf
14. Chen XP, Xiao BQ, Shi W, Xu H, Gao K, Rao J, Zhang Z. Impact of acute vivax malaria on the immune system and viral load of HIV-positive subjects. Chin Med J. 2003;116(12):1810-1820. Accessed on 2007-04-13 at: http://www.cmj.org/Periodical/PDF/2003/2003121810.pdf
One possible conclusion from the table above is that the Heimlich Institute Foundation Inc. sponsored or plans to sponsor malariotherapy trials in countries other than China. It appears that the Heimlich Institute and Deaconess have additional possible clinical trial sites for malariotherapy experiments on subjects with HIV/AIDS in African nations. The 2001 issue of the Heimlich Institute Foundation's newsletter claims that following a meeting with John Gall, chair of the Heimlich Institute board, and professional baseball player, Peter Akinola, Archbishop of the Anglican Church of Nigeria, obtained the support of the National Committee and has secured a physician to head the team and is currently recruiting doctors to participate in the treatment program.
With all due respect, the homophobic archbishop is an inappropriate person to sponsor research of any sort, much less dubious research that's been condemned as unethical and lacking in scientific merit by American regulators. What can these men be thinking? The moment a powerful religious leader is associated with a research project, prospective subjects' spiritual devotion to their church could, and probably would, coerce them into enrolling in research for the wrong reasons. (2)
According to a media article posted on the Heimlich Institute web site, Dr. Adama Sallah, a Rotarian from Gambia, became interested in setting up experiments in his private hospital in Gambia following a 2002 visit with Dr. Heimlich. (3)
The spring 1999 issue of the Heimlich Institute's newsletter describes possible research on malariotherapy for persons with HIV/AIDS proposed by Dr Koos Oosthuizen, M.D., primary occupational health advisor to the Randfontein Estates Gold Mines, in which he and a colleague hoped to enroll 300 subjects with HIV/AIDS. This issue also describes the efforts of the Rt. Reverend Herbert Thompson Jr., Episcopal Bishop of Southern Ohio, to encourage Episcopal bishops in Africa to support experiments with malariotherapy on persons with HIV/AIDS. These scenarios are difficult to defend because clinical investigators and sponsors are obligated to make every effort to avoid situations in which authority and status inequalities might coerce or unduly influence subjects to enroll in research. (4)
Definite plans for expanding malariotherapy research to include subjects with HIV/AIDS in Africa are described in the fall 1999 edition of the Institute's newsletter, and funding in the amount of $1.2 million is solicited to conduct the research. (5)
In the spring 2000 newsletter, the Heimlich Institute solicited research funding for experiments with malariotherapy on human subjects with cancer, which must by now be some sort of record: Dr. Heimlich's involvement with malariotherapy for cancer dates to 1986 (at least), and it's approaching twenty years but he's yet to publish a report of his data. (6)
As of January 2005, the Heimlich Institute Foundation Inc. is still soliciting funds from the public to conduct clinical trials, several of which have been condemned at length by federal regulatory agencies and specialists as unethical, potentially deadly, and in violation of U.S. federal regulations and widely shared ethical principles for the protection of human research subjects. (7)
How to help support lifesaving projects of The Heimlich Institute:
What life-saving medical advances lie just beyond our reach because we lack the funds for research? The goal of The Heimlich Institute is to find simple, creative solutions to complex problems to save and improve lives all over the world. With your support, The Heimlich Institute can continue its important research into treatments for HIV/AIDS, cancer, cystic fibrosis, asthma and many other health conditions. We already have evidence that many of these methods work, but further studies are needed to substantiate these procedures and treatments.
The Institute depends on funds from free-thinking individuals and organizations to continue its commitment to saving lives. Click here for a form you can print out and use to send a gift by check or money order made payable to:
Deaconess Foundation:
for benefit of The Heimlich Institute
and mail to:The Heimlich Institute,
311 Straight St.,
Cincinnati, OH 45219-9957The Heimlich Institute is a non-profit organization. Contributions to support our research are tax-deductible. You may direct your gift to any of the projects listed in this website, or provide undesignated funds to be used where they are most needed. Thank you for your support.
The nonprofit Heimlich Institute is a permanent institution in Cincinnati, Ohio and is directly affiliated with Deaconess Associations Inc. (8)
What's more troubling about Dr. Heimlich's research is the involvement of Deaconess Associations, Inc., the parent corporation of both the Heimlich Institute Foundation Inc. as well as Deaconess Hospital and a number of healthcare corporations. It's difficult to understand why the owner of licensed healthcare facilities might reject federal regulations for the protection of human research subjects and widely accepted ethical guidelines on human research – as is implied by their solicitation of donations to support malariotherapy and other research advocated by Dr. Heimlich.
Does Deaconess Hospital have an institutional review board (IRB)? According to information available from the FDA, the Deaconess Hospital IRB was the IRB of record for six studies conducted under Investigational New Drug applications filed between 1988-05-05 and 2002-04-23. (9)
This information is confusing. The registry of IRBs with a Federal Wide Assurance (FWA) on file with the Office for Human Research Protections (OHRP) has no entry for the Deaconess Hospital IRB. The OHRP registry of FWAs filed by institutions, however, shows that Deaconess Hospital relies on the University of Cincinnati Medical Center IRBs nos. 1A and 1B. While it's possible that the Deaconess IRB no longer exists, it's difficult to understand why Deaconess Hospital would rely on the IRBs at the University of Cincinnati Medical Center if they had their own IRB, and if Deaconess had it's own IRB, why they would choose not to register it with OHRP, and thereby pledge to comply with federal regulations for the protection of human subjects as set forth in the Common Rule at 45 CFR 46. (10)
If Deaconess Hospital has pledged to comply with the Common Rule in research conducted by or at the institution, why wouldn't their parent corporation believe these same basic protections should be afforded to research subjects in China, or Africa (if the studies described above began enrollment), or to subjects elsewhere in the U.S., either enrolled in clinical trials testing the Heimlich Manueover for asthma, cystic fibrosis, or for rescusitation in drowning? What is the basis for this double standard in the protection of research subjects?
It's difficult to reconcile Deaconess Associations' support of Dr. Heimlich's research with the Christian virtues espoused in their statement of Mission and Corporate Values:
Corporate Values
To accomplish our Mission, we hold these Corporate Values as central to our success:Spiritual Values
We embrace and promote the spiritual values of our Christian heritage to guide our ethical decision making and to promote wholeness in our staff and those we serve.Respect
We value an atmosphere of trust and fairness and hold the highest regard for the worth and rights of others.Shared Values is the Corporate Compliance Program at Deaconess Associations that helps all employees to
do the right thing.At Deaconess, our intent is to conduct all of our business in compliance with both the letter and the spirit of applicable rules, regulations, policies and procedures. Our intent is to conduct our business with honesty, integrity and a strong commitment to high ethical standards. (11)
Corporate sloganeering aside, one still wonders why Deaconess Associations offers financial support and publicity for malariotherapy research when regulators and experts have condemned it.
Notes
1. Deaconess Foundation, Immediate Needs. Accessed on 2007-04-13 at: http://web.archive.org/web/20041209011435/http://www.deaconessfoundation.org/needs.html
2. Heimlich Institute's Caring World. Summer 2001. Accessed on 2007-04-13 at: http://web.archive.org/web/20050309105508/http://www.heimlichinstitute.org/media/CaringWorldSummer01.pdf
Archbishop refuses to back down. Stephen Bates. The Guardian. 2004-10-21. Accessed on 2005-06-22 at: http://www.guardian.co.uk/religion/Story/0,2763,1332177,00.html
3. Exchange may help local AIDS research. Gary Presley. Indian Hill Journal. 2002-05-09. Accessed on 2007-04-13 at: http://web.archive.org/web/20050316052530/http://www.heimlichinstitute.org/media/HJHSallah.pdf
4. Heimlich Institute's Caring World. Spring 1999. Accessed on 2007-04-13 at: http://web.archive.org/web/20050309104357/http://www.heimlichinstitute.org/media/CaringWorldSpring99.pdf
5. Heimlich Institute's Caring World. Fall 1999. Accessed on 2007-04-13 at: http://web.archive.org/web/20050309102301/http://www.heimlichinstitute.org/media/CaringWorldFall99.pdf
6. Heimlich Institute's Caring World. Spring 1999. Accessed on 2007-04-13 at: http://web.archive.org/web/20050309103923/http://www.heimlichinstitute.org/media/CaringWorldSpring00.pdf
7. The trouble with Henry. Shane Johnson. Salt Lake City Weekly. 2004-12-30. Accessed on 2005-01-12 at: http://www.slweekly.com/editorial/2004/city_2004-12-30.cfm
Using the Heimlich maneuver in an acute asthmatic condition … could actually kill somebody.
Violations of federal regulations in study titled A Pilot Study of Induced Malaria Therapy in 30 Human Immunodeficiency Virus Positive Individuals.
FDA warning letter to Great Lakes College of Clinical Medicine IRB (2000-03-09) pp. 4, 9-10: http://www.circare.org/foia2/fda_glccm20000309.pdf
8. The Heimlich Institute Foundation Inc., How You Can Help. Accessed on 2007-04-13 at: http://web.archive.org/web/20041028081040/http://www.heimlichinstitute.org/youcanhelp.html
9. According to FDA's Bioresearch Monitoring Information System, the Deaconess Hospital IRB, 311 Straight St. Cincinnati, OH 45219, was the IRB of record for IND applications received by FDA on 5/5/88, 10/24/88, 8/3/93, 3/14/94, 8/30/96, and 4/23/02. Accessed on 2005-06-22 at: http://www.fda.gov/cder/Offices/DSI/BMISlist.htm
10. The Institutional Review Board Registry maintained by the Office for Human Research Protections (OHRP) has no entry for an IRB at Deaconess Hospital in Cincinnati, Ohio. Accessed on 2005-06-22 at: http://ohrp.cit.nih.gov/search/asearch.asp
The OHRP registry of Federal Wide Assurances has an entry for a Federal Wide Assurance for Deaconess Hospital (FWA00008417), which relies on the University of Cincinnati Medical Center IRBs nos. 1A and 1B. Accessed on 2005-06-22 at: http://ohrp.cit.nih.gov/search/asurdtl.asp?ASURID=10959&ASURIDENT=FWA00008417&asutyp=F&asunme=Deaconess+Hosp
11. Deaconess Associations Inc., Mission and Corporate Values. Accessed on 2007-04-13 at: http://web.archive.org/web/20041208210551/http://www.deaconessassociations.com/daimission.html
Last Updated: 2007-04-13
If you find the information on this page helpful, please support CIRCARE with a tax-deductible contribution today. Because CIRCARE doesn't accept funds from pharmaceutical or medical device manufacturers, we depend on contributions from individuals like you to help us advocate for meaningful protection of human subjects in research. Donating on-line with PayPal is quick and easy – find out more on our Support page
All materials on this site © CIRCARE Incorporated (2002- ) or as indicated. Single copies can be downloaded for education. Adobe® Reader :: ::