
 What's Wrong With This Research
What's Wrong With This Research
 Study Documents
Study Documents  Regulatory Correspondence
Regulatory Correspondence
 Funding and Sponsorship
Funding and Sponsorship  Media Articles
Media Articles
 Research Publications
Research Publications
 Research on Malaria and HIV
Research on Malaria and HIV
 Credibility
Credibility  FOIA: UCLA, NIH and John Fahey, M.D.
FOIA: UCLA, NIH and John Fahey, M.D.  Varia
Varia
Editor's comments appear in Arial font gray text.
The simple answer to the question posed by the title of this section might be nearly everything
, but a flippant answer won't help the average reader navigate through federal regulations or the federalese jargon so familiar in the regulatory industry. What makes regulatory-types fall off their chairs is just as likely to put casual readers to sleep, but the issues are important so some introduction and guidance is in order.
The major objection to Dr. Heimlich's tests of malariotherapy on subjects with HIV/AIDS is the lack of data suggesting this would be safe and efficacious, or a plausible mechanism by which it might do so. Widely shared ethical principles prohibit human testing under these circumstances. For example:
Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and on adequate laboratory and, where appropriate, animal experimentation. (7)
Beneficence refers to the ethical obligation to maximize benefits and to minimize harms. This principle gives rise to norms requiring that the risks of research be reasonable in the light of the expected benefits, that the research design be sound, and that the investigators be competent both to conduct the research and to safeguard the welfare of the research subjects. Beneficence further proscribes the deliberate infliction of harm on persons; this aspect of beneficence is sometimes expressed as a separate principle, nonmaleficence (do no harm). (8)
In 1993, senior officials at the CDC wrote a four page memo intended for public release in which they detailed medical, ethical, and scientific objections to Dr. Heimlich's proposal to test malariotherapy on human subjects with HIV/AIDS. Anyone can now read Dr. Heimlich's proposal titled IMT (induced malariatherapy): A Potential Cure for AIDS
, and see why CDC responded against it.
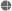 IMT: A Potential Cure For AIDS, A Proposal For Funding, by The Heimlich Institute Foundation, Inc. (1993)
IMT: A Potential Cure For AIDS, A Proposal For Funding, by The Heimlich Institute Foundation, Inc. (1993) 
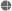 Petition to US Federal Agencies, Public Citizen, and The National Council Against Health Fraud to investigate issues raised by
Petition to US Federal Agencies, Public Citizen, and The National Council Against Health Fraud to investigate issues raised by IMT: A Potential Cure For AIDS
, funding proposal circulated by the Heimlich Institute (1993-05-07) 
 Letters of expert opinion accompanying
Letters of expert opinion accompanying Petition to US Federal Agencies
.
The CDC letter:
Recently the Centers for Disease Control and Prevention (CDC) has received inquiries regarding a proposal titled
IMT (induced malariatherapy): A Potential Cure for AIDS. This proposal has been circulated by the Heimlich Institute Foundation for the purpose of investigating the use of induced malaria infection to cure HIV infection in 10 HIV seropositive patients. Based on our involvement in both ongoing discussions of induced malaria infection for the treatment of Lyme disease in the United States and in assessing the possible interaction between Plasmodium falciparum malaria and HIV/AIDS in subsaharan Africa, we offer the following comments on this proposal:I. Induced malaria infection for neurosyphilis and Lyme disease
Malariatherapy is the induction of symptomatic malaria infection through the inoculation of malaria parasites typically through intramuscular or intravenous injection of blood from a malaria infected donor. Induced malaria infection was used to treat neurosyphilis before the advent of antibiotics. However, controlled studies were never performed to evaluate this mode of treatment, and published reports suggest that the clinical response was unpredictable. Whether or not induced malaria infection was beneficial in the treatment of neurosyphilis remains unknown.
Since 1990, the Heimlich Foundation has encouraged the use of induced malaria infection for Lyme disease in the United States. CDC has officially gone on record opposing this therapy. Reportedly, patients with purported Lyme disease have been injected with blood from individuals in Panama and Mexico infected with Plasmodium vivax. However, induced malaria infection for Lyme disease has never been proven effective; despite reported
cures, there are no published data to show that patients had documented Borrelia burgdorferi infection or that these patients were either cured of their infection or had resolution of clinical signs attributed to Lyme disease.CDC opposes induced malaria infection for the treatment of Lyme disease because: 1) there is no scientific evidence that malaria infection is effective in the treatment of Lyme disease; 2) induced malaria causes iatrogenic morbidity and carries a direct risk for death from complications of P. vivax infection or from co-infection with other, undetected, blood borne pathogens (see III below); and 3) there is a small but finite potential for local transmission of malaria from parasitemic persons in the United States.
…Considerable published experience in Africa, where malaria is hyperendemic and where AIDS has reached epidemic levels, suggests that malaria has no protective effect against HIV infection or the progression to AIDS.
III. Lack of evidence for beneficial effects of malaria infection
No evidence currently exists to indicate that malaria infection would beneficially effect the course of HIV infection, either through induction of fever or alterations in immunologic parameters. The perceived benefit of malaria infection for neurosyphilis was believed to be attributable to the destruction of the causative spirochete by repeated high fevers (40–42° C). Induced fever is unlikely to have any benefit in the treatment of HIV/AIDS since temperatures in this range have little effect on the HIV virus. In 1990, the National Institute of Allergy and Infectious Disease (NIAID) investigated the use of systemic hyperthermia for the treatment of HIV/AIDS and concluded there was no basis for the use of hyperthermia in the treatment of HIV disease.
…V. Ethical Considerations
Any protocol involving the use of humans in research should undergo thorough ethical review and approval by a human subjects review board prior to initiation.
Without evidence, either in-vitro or in-vivo, to support the hypothesis that malaria suppresses HIV infection or delays the development of AIDS, and with the risk of adverse health consequences associated with induced P. vivax infection or other blood borne pathogens, the use of induced malaria infection in HIV infected individuals can not be justified. (1)
Based on their inspection of the Great Lakes Association of Clinical Medicine Institutional Review Board, FDA suspended their authority to review and approve new studies or enroll new subjects in research subject to FDA regulation, and following nine months of unsatisfactory responses from the IRB, the agency terminated seven clinical trials (3). What follows are FDA's objections to violations of federal regulations for the protection of research subjects by the Great Lakes Association of Clinical Medicine IRB in its review and approval of Dr. Heimlich's study titled Induced Malaria as Therapy for HIV Infection
. Because this research was privately funded and conducted outside the US, the FDA lacked jurisdiction to take administrative action against Dr. Heimlich as well, but many people would agree that the matters complained of – violations of codifications of basic ethical principles – pertain as well to the study sponsor and author of the trial protocol and informed consent, namely Dr. Heimlich and the Heimlich Institute Foundation Inc.
Failure to prepare detailed written procedures for conducting the review of research, including periodic review. [21 CFR 56.108(a), 56.l15(a)(6) ]
…The IRB procedures should define whether the IRB will review proposed research to be conducted only in foreign countries, and whether there should be additional procedures when the proposed research is only conducted out of the United States. The IRB approved the study titled
Induced Malaria as Therapy for HIV Infectionconducted in China. The IRB did not review the Chinese translation of the protocol or consent form, and has no information about how subjects and malaria parasite donors are recruited and screened. See item 2, 6, and 11, below. (4)
Failure to consider community attitudes and cultural backgrounds. [21 CFR § 56.107(a) ]
The IRB reviewed and approved the study titled
Induced Malaria as Therapy for HIV Infectionconducted only in China. There is no documentation as to how the IRB considered the local community attitudes or cultural attitudes towards two of the significant aspects of the research: the direct injection of blood from one person into another person, and that the subject will be administered live malaria parasites. Please explain how the IRB determined that this research was acceptable for Chinese citizens. Would the IRB's approach have been different if the research was conducted in the United States? (5)
Failure to require that information given to subjects as part of informed consent is in accordance with the provisions of 21 CFR § 50.25. [21 CFR § 56.109(b) ]
The IRB approved consent forms that do not meet federal regulations. The consent forms submitted by Dr. Page and Dr. Heimlich and approved by the IRB are representative examples:
…The English version of the consent form for the study titled,
Induced Malaria as Therapy for HIV Infectionis deficient for the following reasons (this is not a complete list):1. The consent form does not adequately describe the procedures to be followed. The consent form states,
Natural infusion of malaria parasites will be administered on day 1.The actual procedure involves injection of blood from a malaria-infected person into the study subjects. There is no description of the steps taken to screen malaria parasite donors for pathogens.2. The duration of the study is described as
unlimited.The long-term risks of the study and the frequency of follow up are not defined.3. The risks of receiving blood from another person are not described. The possibility of receiving blood-borne pathogens is not discussed.
4. There is no description of the consequences of a subject's decision to withdraw from the research, such as during the stage of malaria infection.
5. There is no description of the lifelong risks associated with malarial infection, other than ruptured spleen and death.
6. The consent form lacks the identity of whom to contact in the event of research-related injury to the subject.
7. The consent form lacks an explanation of whom to contact for answers to questions about research subjects' rights.
8. Use of the wording
You understand..is inappropriate. The subjects may certify that they understand the statements in the consent form and are satisfied with the explanation provided by consent process, but many will not comprehend the underlying scientific and medical significance of all the statements, nor are they in a position to judge whether the information provided is complete. Subjects should not be required to certify such understanding or completeness of disclosure.9. The name of the clinical investigator is indicated only by
XXXXXX.The IRB should know the identity of the person conducting the study.10. The consent form contains exculpatory language in which the prospective subject is made to waive or appear to waive any of the subject's legal rights, or releases or appears to release the investigator, the sponsor, the institution, or its agents from liability for negligence. (6)
In response to FDA's request to explain how the IRB determined that this research was acceptable for Chinese citizens
, and if the IRB's approach [would] have been different if the research was conducted in the United States
, Dr Chappell made this reply:
The IRB required Dr. Heimlich to present his proposed research project in person. IRB members questioned Dr. Heimlich for at least 45 minutes on the history of this procedure, (it was used as a treatment for syphilis prior to antibiotics), the precautions to be taken, the recruitment of subjects, the qualifications of the facility to be utilized and other pertinent issues. The IRB was very comfortable that the potential benefit for subjects was far greater than the risk of this therapy and that this research was acceptable for Chinese citizens. Since his work was associated with an academic institution in China that commonly does international research, we assumed that the community standards and the consent translation were adequate. In the future, we will contact the foreign institution to check this. Our IRB's approach was no different than if the research was conducted in the U.S. (10)
Dr. Chappell's reply drew a sharp response from FDA:
7. We have the following comments regarding the IRB's review and approval of the study entitled
Induced Malaria as Therapy for HIV Infection.We strongly disagree that the IRB properly considered the scientific merit of the study and that the protocol minimized risks to subjects concerning this study, as expressed in you response letter dated March 17, 2000, for the following reasons:A. The protocol is inadequate in that it does not describe what testing was done to screen the malarial parasite donors. The direct injection of blood from a malaria parasite donor into a study subject would not be permitted in the U.S. because cultured malaria parasites are available. The IRB did not review information about how subjects and malaria parasite donors are recruited and screened.
B. The statement in the response letter that
the IRB's approach [to the study] was no different than if the research was conducted in the U.S.demonstrates that the IRB appears to lack the expertise or experience to ascertain the acceptability of proposed research in terms of institutional commitments and regulations, applicable law, and standards of professional conduct and practice.Page 10 – Great Lakes College of Clinical Medicine IRB
C. We reject your position that the IRB was able to consider the community attitudes of the Chinese population in which the research was to be conducted. Given the great differences between Chinese and American cultures, we do not accept that the GLCCM was capable of understanding Chinese attitudes about the research. This is one reason, among many, that the academic institution in China that was associated with the research, as referenced in your response letter, should have obtained governmental and approval from a local IRB (or equivalent body).
D. Although Dr. Heimlich's Foundation is apparently underwriting this research study, he has no responsibilities for subject screening, study procedures, or evaluation of the subjects, and appears to have no direct supervisory role over the study. Dr. Heimlich is not obligated to obtain IRB approval for his limited involvement in this study, and, indeed, in this case, it was inappropriate for him to do so.
E. The IRB file for this study did not contain the Chinese translation of the protocol or consent form. In general, when study subjects are non-English speakers, the IRB must assure that the consent form translation is accurate.
F. Please describe the IRB's efforts to determine that this study had been approved by the appropriate office in the Ministry of Health and by the local institution(s) where the research was conducted. The IRB should rescind approval of this study and defer the human subject protection responsibilities to the responsible Chinese authorities.
Please provide documentation that the IRB has informed the Chinese clinical investigator of his responsibility to obtain the appropriate Chinese government and local institutional approval for their research. (11, with emphasis added)
Whereupon Dr. Chappell replied:
We have requested the name of the Chinese clinical investigator from Dr. Heimlich to inform the investigator of his responsibility to obtain the appropriate Chinese government and local institutional approval for the research. (12)
After receiving Dr. Chappell's response of 2000-05-26, FDA quit writing to him and addressed themselves to Jack Hank, executive director of the Great Lakes College of Clinical Medicine. Perhaps part of the impetus for their action is evident from this complaint raised with Mr. Hanks, regarding the IRB's most recently submitted revision of their Standard Operating Procedures:
The purpose of the reference to
community attitudesin the regulations is to assure sensitivity to such issues in IRB review of proposed research, not to determine whether thecommunity attitudes, laws, and morals are more restrictive than Federal requirements.Federal law does not mandate morals and community attitudes. Please revise. (13, with emphasis added)
Notes
1. Chief, Malaria Branch, DPD NCID, Associate Director for Science, HIV NCID, Chief, Bacterial Zoonoses Branch, DVBID NCID, US Centers for Disease Control, comments on IMT (induced malariatherapy): A Potential Cure for AIDS
, proposal circulated by The Heimlich Institute Foundation. (1993-04-29) 
2. Document Index at: http://www.circare.org/foia2/docs.htm
3. Suspension of authority: FDA Warning Letter to Great Lakes College of Clinical Medicine IRB, L. Terry Chappell, M.D., Secretary (2000-03-09), p.13  ; termination of studies: FDA to Great Lakes College of Clinical Medicine IRB, Barbara Grunewald, Executive Director (2001-01-02), p.2.
; termination of studies: FDA to Great Lakes College of Clinical Medicine IRB, Barbara Grunewald, Executive Director (2001-01-02), p.2. 
4. FDA Warning Letter to Great Lakes College of Clinical Medicine IRB, L. Terry Chappell, M.D., Secretary (2000-03-09), p. 2–3. 
5. FDA Warning Letter to Great Lakes College of Clinical Medicine IRB, L. Terry Chappell, M.D., Secretary (2000-03-09), p. 4. 
6. FDA Warning Letter to Great Lakes College of Clinical Medicine IRB, L. Terry Chappell, M.D., Secretary (2000-03-09), p. 7, 9–10. 
7. World Medical Association, Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects; B. Basic Principles for All Medical Research
, 11. Accessed 2005-01-03 at: http://www.wma.net/e/policy/b3.htm
8. International Ethical Guidelines for Biomedical Research Involving Human Subjects, CIOMS Council for International Organizations of Medical Sciences (November 2002); General Ethical Principles
. Accessed 2005-01-03 at: http://www.cioms.ch/frame_guidelines_nov_2002.htm
9. Henry Heimlich, M.D., to Robert L. Kaiser, M.D., Director, Division of Parasitic Diseases, Centers for Disease Control, re: update on malariatherapy for cancer. (1987-07-22) 
10. L. Terry Chappell, M.D., Secretary, Great Lakes College of Clinical Medicine IRB, to FDA (2000-03-17), p. 3. 
11. FDA to Great Lakes College of Clinical Medicine IRB, L. Terry Chappell, M.D., Secretary (2000-04-13), p. 9-10. 
12. L. Terry Chappell, M.D., Secretary, Great Lakes College of Clinical Medicine IRB, to FDA (2000-05-26), p. 2. 
13. FDA to Great Lakes College of Clinical Medicine IRB, Jack Hank, M.D., Executive Director (2000-06-21), p. 3. 
Last Updated: 2007-04-01
If you find the information on this page helpful, please support CIRCARE with a tax-deductible contribution today. Help us advocate for meaningful protection of human subjects in research. Donating on-line with PayPal is quick and easy – find out more on our Support page.
All materials on this site © CIRCARE Incorporated (2002- ) or as indicated. Single copies can be downloaded for education. Adobe® Reader :: ::